- Highclere Court, Woking, Surrey, GU21 2QP, UK
Abstract
Synaptic
plasticity confers environmental adaptability through modification of
the connectivity between neurons and neuronal circuits.
This is achieved
through changes to synapse-associated signaling systems and supported
by complementary changes to cellular morphology and metabolism within
the tripartite synapse.
Mounting evidence suggests region-specific
changes to synaptic form and function occur as a result of chronic
stress and in depression.
The prefrontal cortex (PFC) and hippocampus
represent the best studied regions where functional and structural
findings are consistent with a deficit in long-term potentiation (LTP),
and neuronal and glial growth at excitatory synapses.
Correlating these
changes may be those to glutamate receptors (AMPARs and NMDARs), growth
factor signaling (BDNF-TrkB) and several signal transduction pathways
(NOS-NO, cAMP-PKA, Ras-ERK, PI3K-Akt, GSK-3, mTOR and CREB).
In contrast
other brain regions such as the amygdala may feature a somewhat
opposite synaptic pathology including reduced inhibitory tone.
Deficits
in synaptic plasticity may further correlate disrupted brain redox and
bioenergetics in stress and depression.
Moreover, at a functional level
region-specific changes to synaptic plasticity in depression may relate
to maladapted neurocircuitry and parallel reduced cognitive control over
negative emotion.



 The
event started out with a panel discussion lead by Dr. Jon Lapook of the
CBS Evening News. The members of the panel were: Dr. Sujana
Chandrasekhar from New York Otology, Dr. Ed Rubel from the University of
Washington, Dr. Stefan Heller from Stanford University and Dr. Andy
Groves from Baylor College of Medicine. An interesting perspective from
the panel discussion was how much less the NIH funds hearing research
compared to cancer and other significant health issues. That was an
important insight into possibly why it is even more difficult to get
funding for hyperacusis since it represents only a fraction of the
population with ear issues.
The
event started out with a panel discussion lead by Dr. Jon Lapook of the
CBS Evening News. The members of the panel were: Dr. Sujana
Chandrasekhar from New York Otology, Dr. Ed Rubel from the University of
Washington, Dr. Stefan Heller from Stanford University and Dr. Andy
Groves from Baylor College of Medicine. An interesting perspective from
the panel discussion was how much less the NIH funds hearing research
compared to cancer and other significant health issues. That was an
important insight into possibly why it is even more difficult to get
funding for hyperacusis since it represents only a fraction of the
population with ear issues.  The
key take-away is that while barriers remain large, the team felt
overall that a real pathway is possible in the next decade or two. The
most important aspect that relates to hyperacusis is that this research
work will continue to drive the need for more detailed understanding of
every inner ear component down to the molecular level. Every new piece
of knowledge gained may further advance possible models relating to
hyperacusis as long as we are connected to the work. For example, a key
issue with hyperacusis is getting solid evidence for whether or not
hyperacusis patients have hair cell damage. Currently all methods to
truly establish hair cell damage can only be done on a destroyed
cochlea. In a follow-up discussion, Dr. Heller described that recent
advances in optical tomography may soon enable this diagnostic tool to
be able to view inside a live cochlea. That breakthrough would enable
many in the study of the inner ear to gain much needed understanding for
modeling the inner ear and help to finally answer the question for
damage for hyperacusis patients.
The
key take-away is that while barriers remain large, the team felt
overall that a real pathway is possible in the next decade or two. The
most important aspect that relates to hyperacusis is that this research
work will continue to drive the need for more detailed understanding of
every inner ear component down to the molecular level. Every new piece
of knowledge gained may further advance possible models relating to
hyperacusis as long as we are connected to the work. For example, a key
issue with hyperacusis is getting solid evidence for whether or not
hyperacusis patients have hair cell damage. Currently all methods to
truly establish hair cell damage can only be done on a destroyed
cochlea. In a follow-up discussion, Dr. Heller described that recent
advances in optical tomography may soon enable this diagnostic tool to
be able to view inside a live cochlea. That breakthrough would enable
many in the study of the inner ear to gain much needed understanding for
modeling the inner ear and help to finally answer the question for
damage for hyperacusis patients.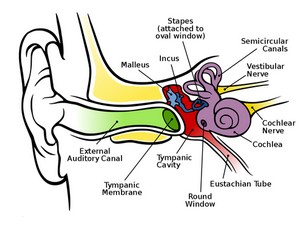 Observing without destroying
Observing without destroying 

